Atoms
An introduction to the basic building
blocks of all matter
All matter is made of atoms, you, me, whatever device you're reading this on, the air through which you're looking at this, Mount Everest and spiderman (ok, spiderman isn't real and therefore isn't made of atoms, so not spiderman).
Atoms are the smallest particle of pure matter that can exist on its own and is stable (there are smaller bits but they go around causing trouble as they aren't stable).
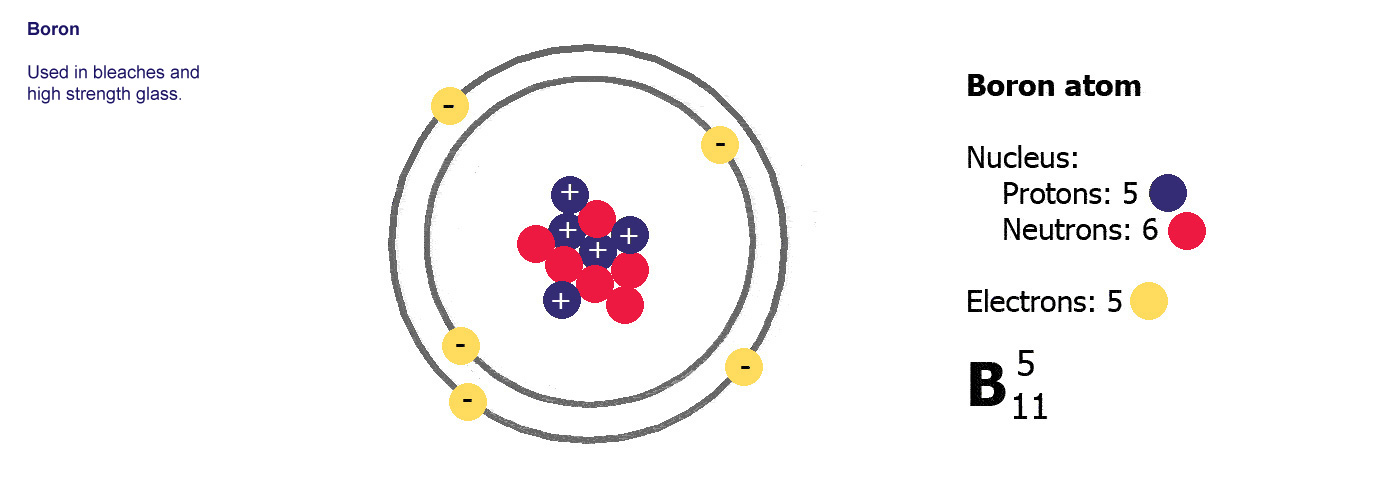
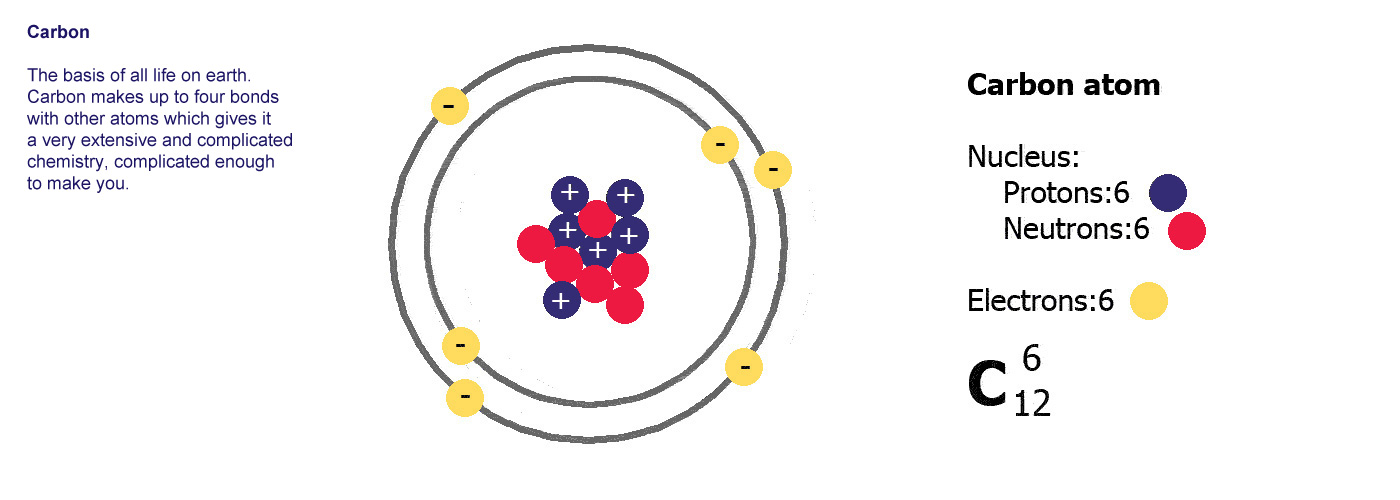
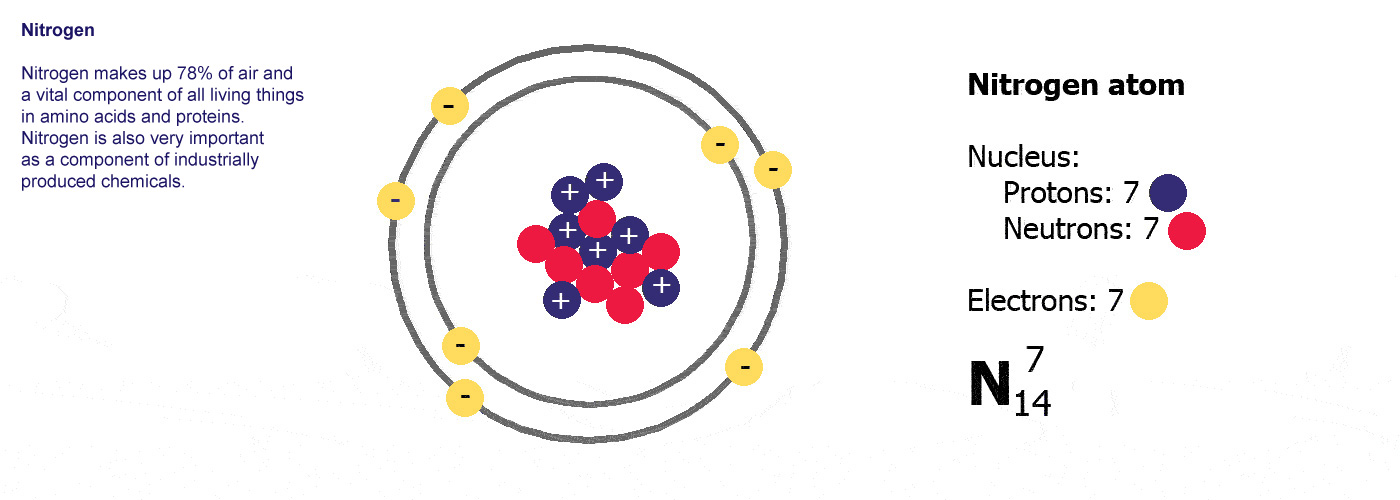
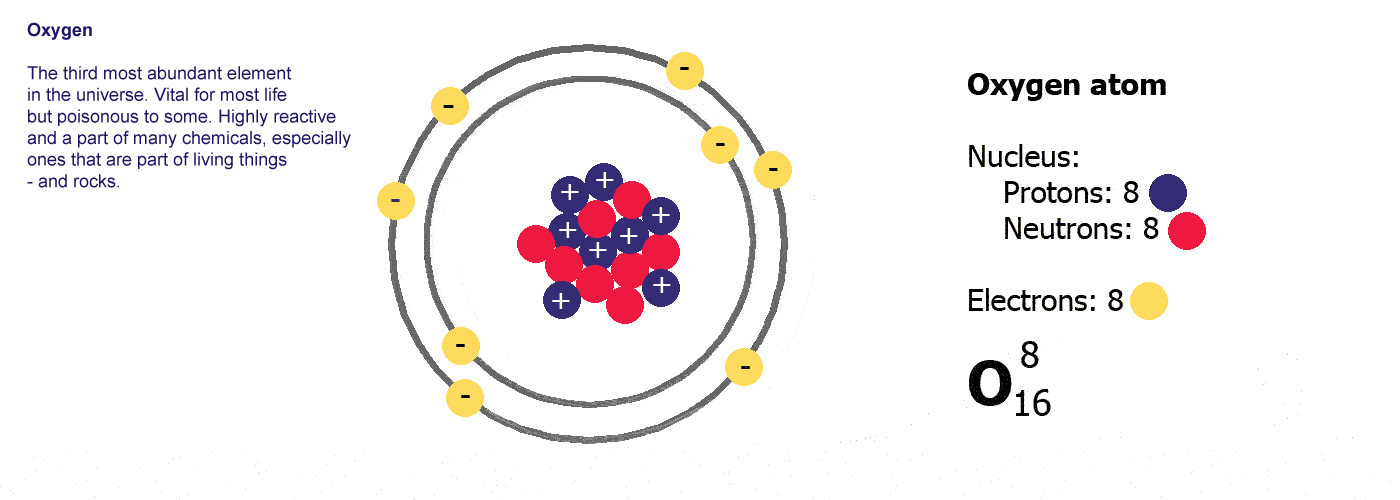
Atoms are made of three basic parts called protons, electrons and neutrons. They are all made of these same basic parts, they differ in how many of them they have. Atoms have the same numbers of protons as they do of electrons, the neutrons are usually a similar number, sometimes the same but not always.
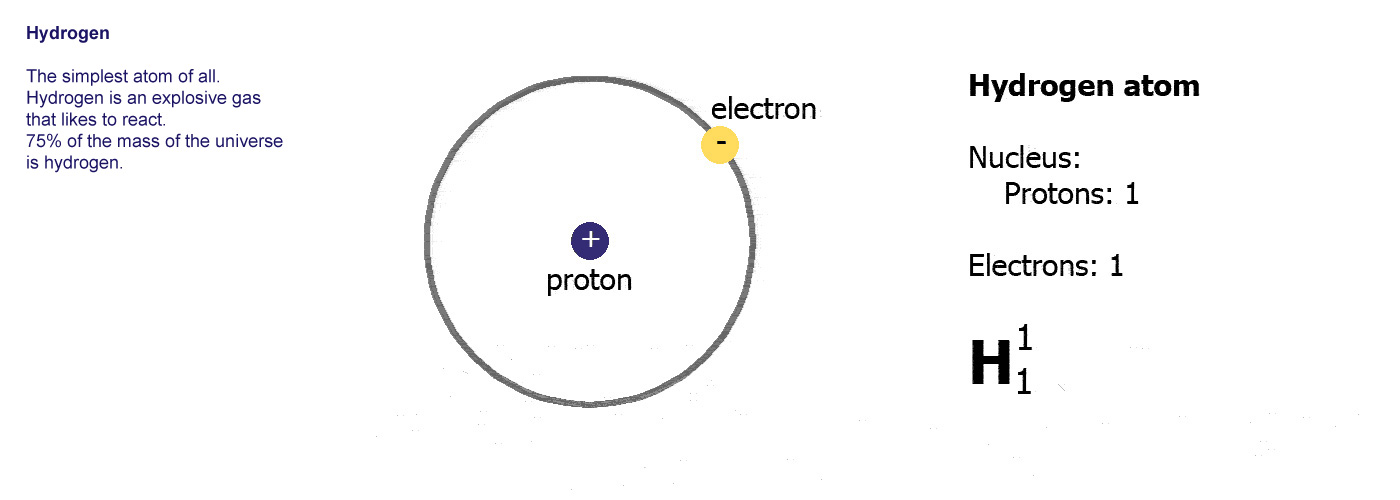
The protons and neutrons are clumped together in the middle of the atom in a region called the nucleus. The electrons fly around the outside as if they were in orbit in a series of shells. The simplest atom is hydrogen (below left) it has one proton and one electron.
Protons are positively charged (p-proton-positive, it's almost logical). Electrons are negatively charged (e-electron-negative, that one didn't work so well did it? you'll just have to remember it). The atom itself has an overall neutral charge as plus 1 and minus 1 makes zero. A charge of zero makes for a happy and calm atom.
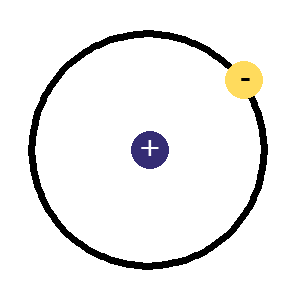 A
hydrogen atom,
A
hydrogen atom,the simplest, 1 proton in the nucleus and an electron orbiting around it.
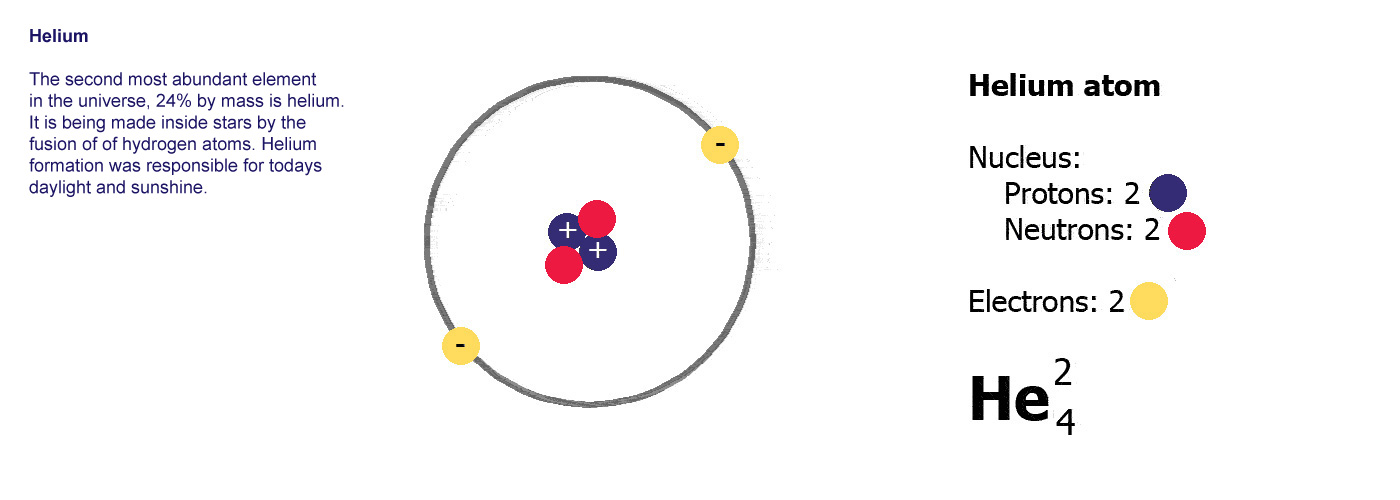
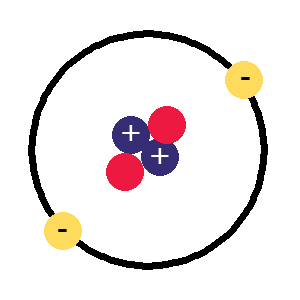 A
Helium atom,
A
Helium atom,the next one up, 2 protons, 2 electrons and now 2 neutrons to stop the protons flying apart
Now if we have two protons, we need two electrons to cancel them out and get a charge of zero, so this is what the next kind of atom has. There's now a problem as that nucleus has two positively charged protons stuck together and they aren't very happy about it, preferring to fly apart as their equal positive charges should make them do so.
This is where the neutrons come in, these have a neutral charge and fulfill the job of keeping the two positively charged protons together instead of flying apart. The next kind of atom up from hydrogen therefore has two protons, two neutrons and two electrons, it is a helium atom (above right).
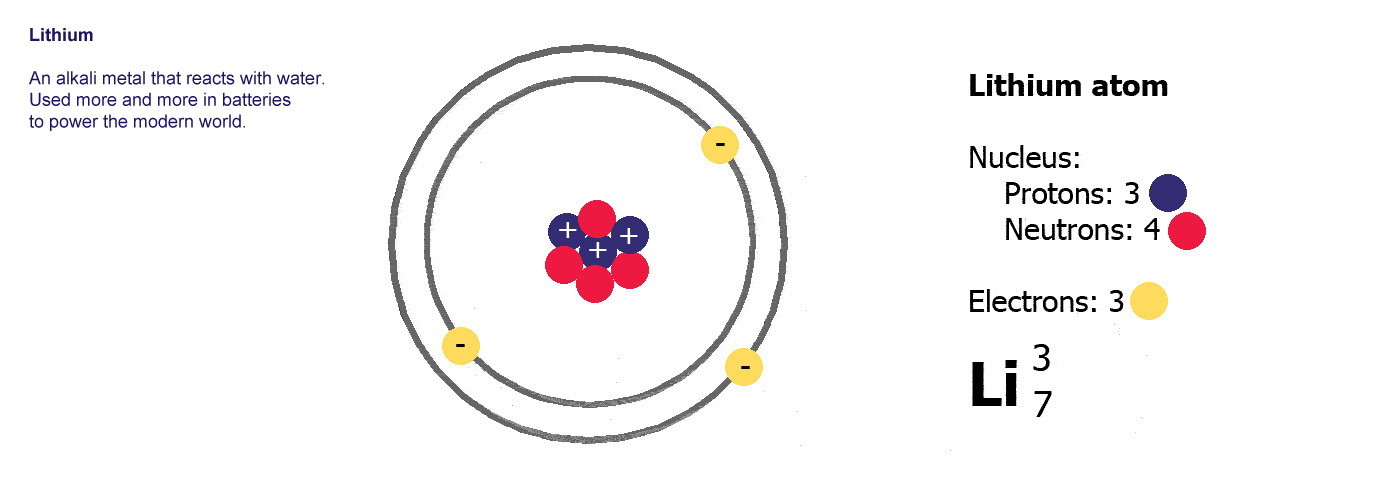
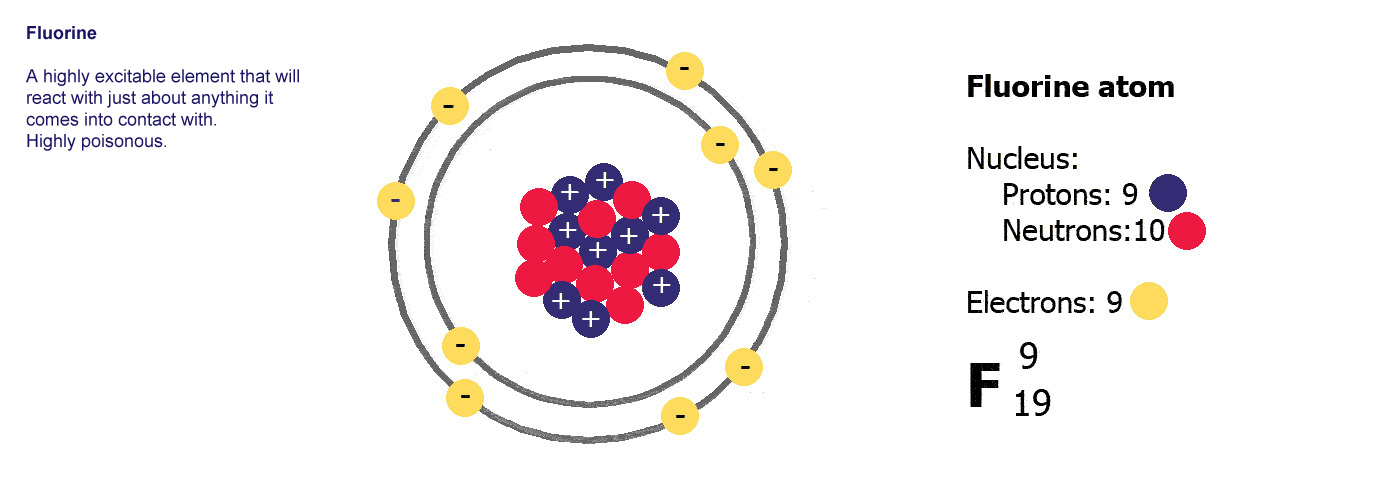
Different kinds of atom like this with different numbers of protons are called elements. An element is defined by the numbers of protons it has, hydrogen always has one, helium always has two and so on, there are diagrams of the first ten elements on the slider at the top of this page. The number of protons is referred to as the atomic number. If you change the number of protons you change the element, so elements always have the same number of protons in the nucleus of the atoms.
Heaviness
The three particles in atoms have quite distinct masses.
Protons and neutrons (the things in the nucleus) both have a designated mass of 1. Electrons are much, much lighter, being about 1/1840th of the mass of a proton or neutron. Even when you add them all together they don't come to much and make as much difference as can be ignored to the overall mass of the atom.
So far we have:
| Particle |
Charge |
Mass |
| Proton | Positive | 1 |
| Neutron | Neutral | 1 |
| Electron | Negative | 0 - (1/1840 really) |
The overall mass of an atom is the sum of its particles added together.
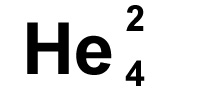 He
is the symbol for Helium. You may see it written
with the numbers as it is to the left. The smaller number, 2
in this case, tells us how many protons there are, the
larger number 4, tells us how many neutrons and protons
there are added
together in the nucleus. We are given the number of protons
which define what the atom is, and then the mass of the
atom. The number of neutrons is worked out by taking the
smaller number (protons) from the bigger number (total mass)
i.e. 4-2 = 2 neutrons, in this case there are the same which
is fairly common but not always true.
He
is the symbol for Helium. You may see it written
with the numbers as it is to the left. The smaller number, 2
in this case, tells us how many protons there are, the
larger number 4, tells us how many neutrons and protons
there are added
together in the nucleus. We are given the number of protons
which define what the atom is, and then the mass of the
atom. The number of neutrons is worked out by taking the
smaller number (protons) from the bigger number (total mass)
i.e. 4-2 = 2 neutrons, in this case there are the same which
is fairly common but not always true.
The proton number is the atomic number, the total number of protons and neutrons is the mass number or atomic mass.
If you remember from further up the page, there are always the same number of electrons as there are of protons in an atom, so we also know there are two electrons in helium.
Sometimes you'll see the bigger number is at the top and smaller one at the bottom, sometimes it's the other way round, it doesn't matter though as the small number is always the protons and the larger number always the total mass.
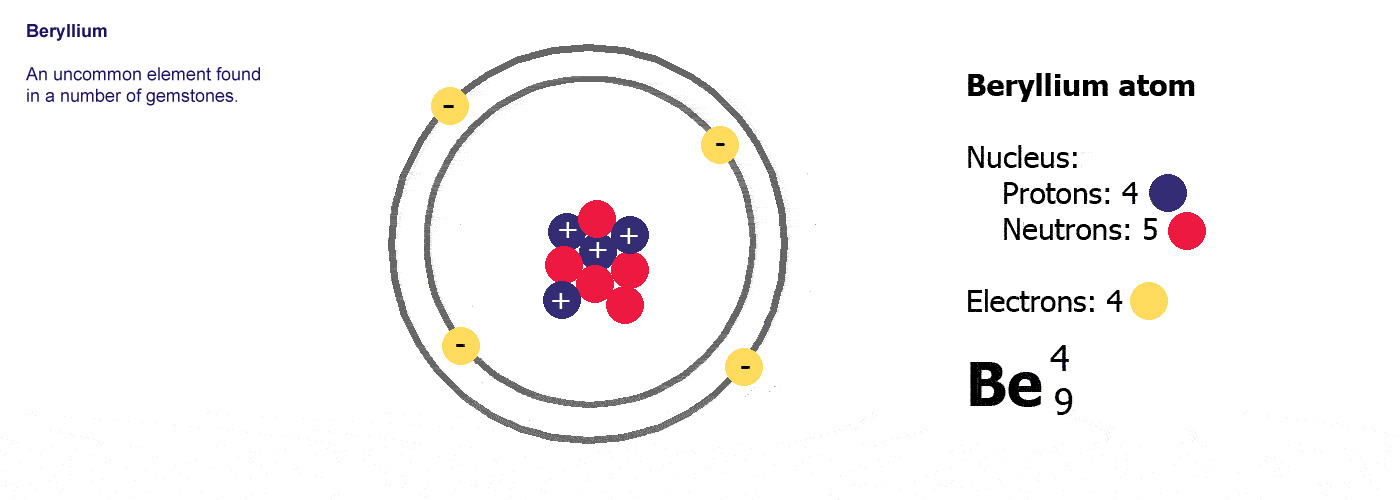
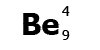 Be is
the symbol for the element beryllium. Atomic number
4, mass number 9.
Be is
the symbol for the element beryllium. Atomic number
4, mass number 9.
Beryllium has 4 protons and a
mass of 9. It has 4 electrons (the same as the protons) and
9-4=5 neutrons.
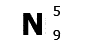 N is
the symbol for nitrogen. Atomic number 5, mass number
9.
N is
the symbol for nitrogen. Atomic number 5, mass number
9.
Nitrogen has 5 protons and a mass of 9. It has
5 electrons (the same as protons) and 9-5=4 neutrons.
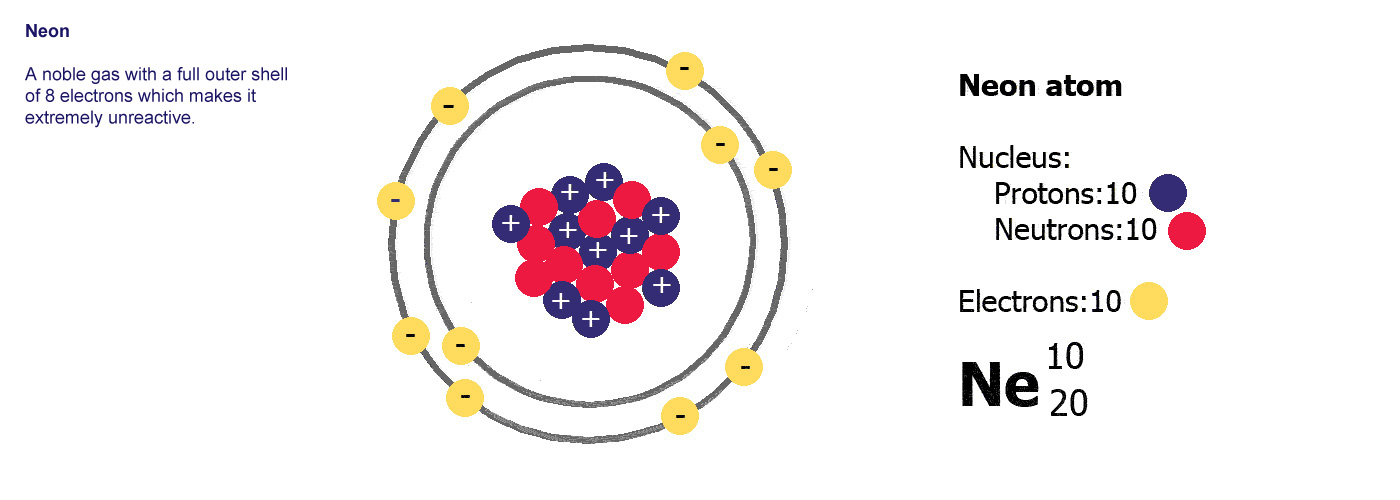
 Ne is
the symbol for neon. Atomic number 10, mass number 20.
Ne is
the symbol for neon. Atomic number 10, mass number 20.
Neon has 10 protons and a mass of 20. It has 10 electrons (the
same as protons) and 20-10=10 neutrons.