Chemistry for Students
School science, chemistry
for 11 to 18's
Chemistry is the study of matter.
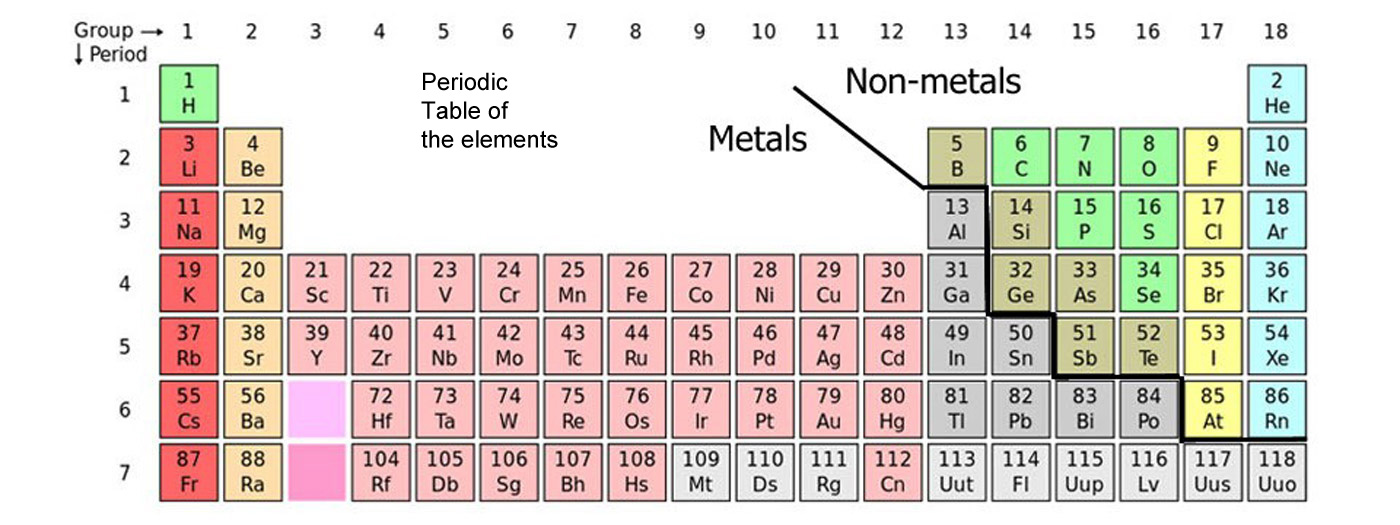
It is the study of the way that matter interacts and makes new substances. The obvious place to start chemistry is with the elements, the simplest pure substances that can be obtained with widely varied properties though they are made of the same three basic particles. Their different properties being derived from having different numbers of these three basic particles, electrons, protons and neutrons.
The simplest commonly found form of matter are pure elements found as atoms, though very few elements have atoms that are happy to hang around on their own. Far more commonly atoms are joined together in molecules that can be made of one or more kinds of element. Other than a few rare (ish) gases, most matter is made of molecules.
Molecules
These are made of more than one atom chemically bonded together, these atoms may all be the same if the substance is an element, or they may have more than one kind of atom when they are called compounds. Most things you are familiar with are made of compounds or combinations or mixtures (not chemically bonded) of compounds, such as you and your computing device you are reading this on for instance.
Chemistry has built much of our modern world.
It has enabled us to develop new materials and combinations of materials to enable us to do things we couldn't do at all previously, or easier and quicker ways of doing things that used to be difficult or only possible in small quantities.
There are chemicals such as coatings, paints, dyes, adhesives and others that change the properties of natural materials in useful ways. Amongst the earliest examples of this are soap and copper made from simple chemical reactions of easily found natural substances. The tanning of leather is another early example of practical applied chemistry that dates back possibly as far as 7000 BC and originally used large quantities of urine and dung water (yes that is exactly what it sounds like) that has been improved in many ways by the development of (less smelly) and more effective chemical methods.
Another early example comes from the the invention of the Bessemer steel converter in the 1850's in Sheffield, England. This allowed high quality steel to be produced after half an hour of processing in quantities of 5 tons and above at a cost of about GBP £7 a ton, previously it took a week to make small quantities at £40 a ton. This was due to a change in the understanding of the chemistry of refining steel allowing the process to be scaled up very significantly. A drop in price to about 1/6th of the previous amount, production in a fraction of the time (about 1%) and at greater quantities (about 250 times per batch) accelerated the progress of the industrial age.
A more recent example is provided in modern electronics including the fancy high quality display you are reading this on. Such displays are dependent on the use of a number of rare earth elements such as Europium, Yttrium and Terbium. Neodymium is used in the manufacture of powerful magnets in things as diverse as the vibrate mechanism in your phone, your ear-bud speakers and wind turbines.
First come the materials in quantities large enough to be widely used, often for a particular original purpose, and then follow new and novel ways to use them - all the results of chemistry.
Chemistry is used at the other end of the size scale too. Analyzing the tiniest amounts of substances found as traces in forensic analysis of crime scenes and archeological remains, of air samples trapped as bubbles in ice cores thousands of years old, of proteins in biological systems and medicine and of DNA sequencing and fingerprinting to name but a few.
There are a whole range of chemical techniques to measure these tiny amounts of substances, such as chromatography which separates mixtures in a gas or liquid solvent and then detects what is there by the color or other measureable characteristics. Spectroscopy detects elements and compounds by measuring what is emitted when the sample is "excited". A simple example of the spectroscopic idea is neon lighting where an electrical current causes neon gas to emit a characteristic color of light used in the light-up letters sometimes used as signs.



 A flask of copper sulphate solution
A flask of copper sulphate solution 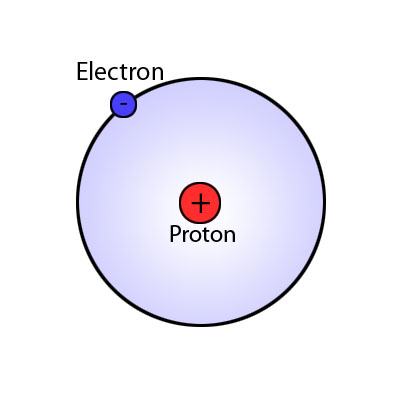 Hydrogen atom
Hydrogen atom  Copper being detected ina flame test
Copper being detected ina flame test 
 Mercury, a metal that is liquid at room temperature
Mercury, a metal that is liquid at room temperature