Chemical Elements
Pure substances
made of just one kind of atom defined by the number of protons
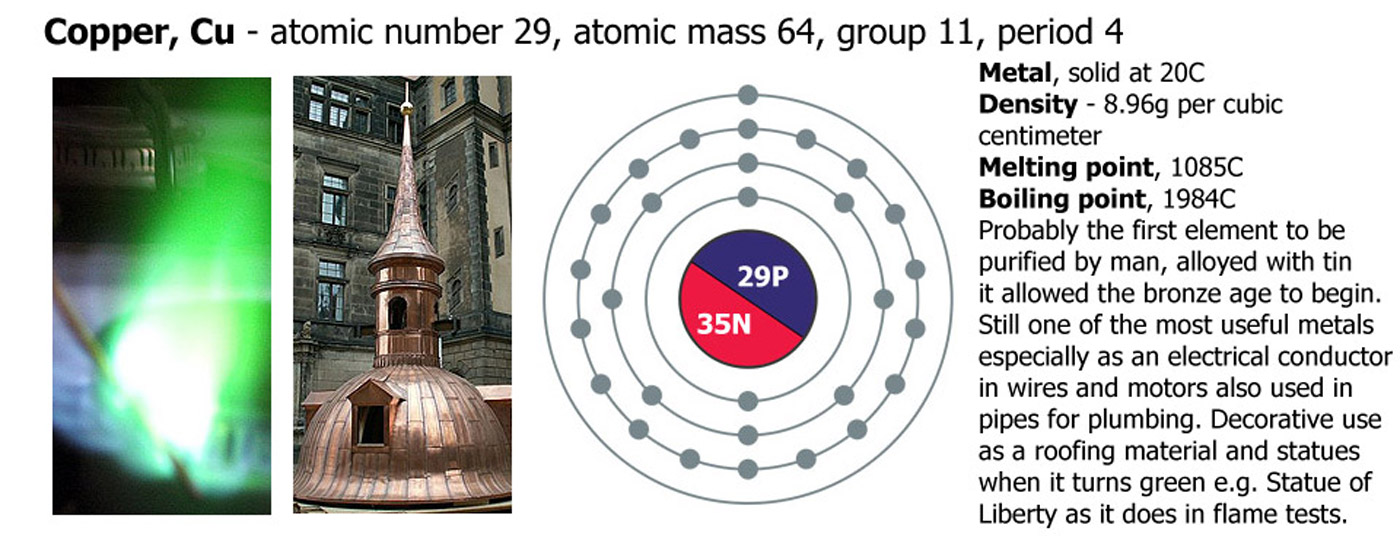
Chemical elements are the raw materials of chemistry and the universe. It was realized from ancient times that some materials could not be further separated or purified. The first elements to be separated (by today's definition of element that is, the ancients had other ideas) were metals such as copper, gold, silver, lead, tin and iron. Copper was the first metal to be extracted from its ore, later on tin was discovered and they were mixed while molten to make bronze signifying the start of the Bronze Age, later on iron smelting was developed which started the Iron Age.
The technological development of mankind is in some ways quite tightly connected to our discovery of new elements and how to obtain them in useful quantities from substances that contain them in impure form.
The different elements all have the same structure in that they are made of the same kinds of atoms. Different elements have different numbers of protons, how many protons they have is referred to as the atomic number, it is the most important thing about an atom and decides what element it is. You can change the number of neutrons or electrons in an atom (to some degree) and will always have the same element, but change the proton number and you get a different element altogether. The new element you get can be enormously different to the next one on or back in proton number terms, with just the one more or less proton making the difference.
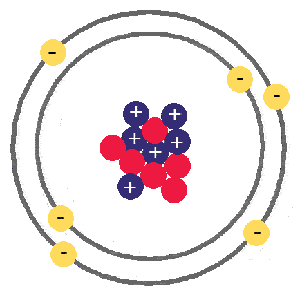
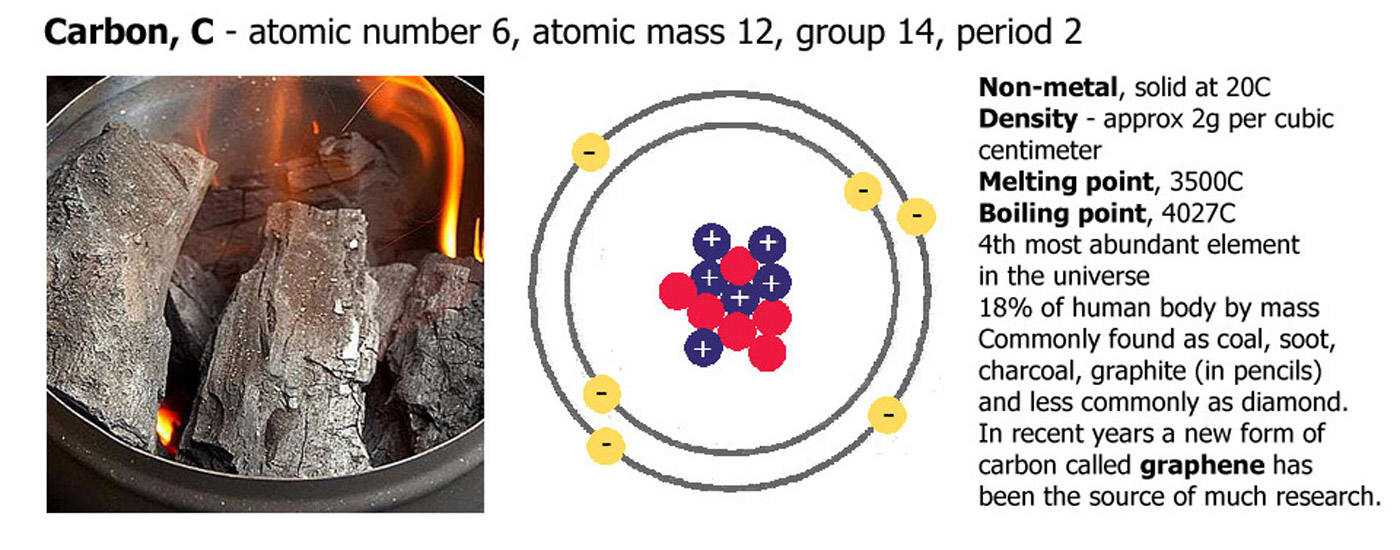
Carbon, atomic number 6, atomic mass 12
Carbon has an atomic number of 6 because it has 6 protons (blue. positive), it also has 6 electrons (yellow, negative), and in this case 6 neutrons (red, neutral). The numbers of neutrons and electrons can change a little and it will still be carbon, but if the proton number changes it will become a different element.

Carbon is a black solid at room temperature
it has a density of around 2g per cubic centimeter. melting point 3500C, boiling point 4027C
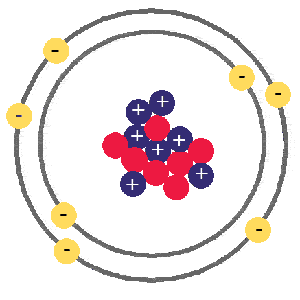
Nitrogen, atomic number 7, atomic mass 14
almost identical to the carbon atom above, but this time it has an atomic number of 7 because it has 7 protons, it also has 7 neutrons and 7 electrons.

Having this extra proton gives nitrogen very different properties.
Nitrogen is a colorless, odorless gas at room temperature, with a density of 0.00125g per cubic centimeter, it turns to a solid at -210C and boils at -198C, all very different to its neighbour carbon.
By the 1800's scientists were able to measure the elements so far discovered by weight, they didn't know about protons, neutrons and electrons but these were effectively what they were measuring. They were puzzled because there didn't seem to be any particular pattern to the elements that had been discovered so far. Like the example of carbon and nitrogen above that differ in just a single proton, elements that might be expected to be similar could actually be completely different.
 It
was a Russian scientist called Dmitri Ivanovich Mendeleev
who gave some sort of explanation to the apparent haphazard
collection of elements that was building up when he developed
the idea of what has become refined into the periodic table
of the elements we use today, in a book he wrote in 1868-1870.
It
was a Russian scientist called Dmitri Ivanovich Mendeleev
who gave some sort of explanation to the apparent haphazard
collection of elements that was building up when he developed
the idea of what has become refined into the periodic table
of the elements we use today, in a book he wrote in 1868-1870.
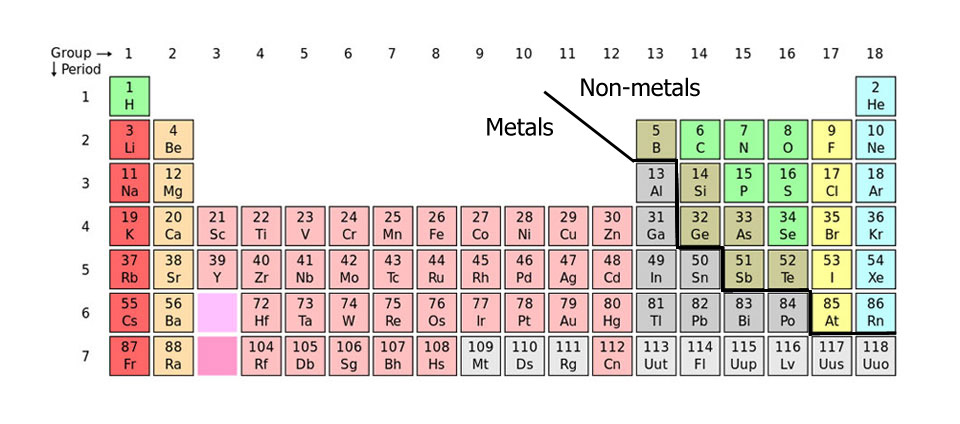
The most important aspect of Mendeleev's table was that the elements were arranged in Groups (vertical) and Periods (left to right) of which the groups are more important for predicting the properties and reactions of an element. So if you look at the table above, you will see that carbon (C, atomic number 6) will have more in common with silicon (Si, 14) and germanium (Ge, 32) than with nitrogen (N, 7) next to it. Likewise nitrogen will have more in common with phosphorous (P, 15) and arsenic (As, 33) than with carbon.
Mendeleev's table like many new scientific ideas wasn't widely accepted at first, though became so when spaces he had left for the then undiscovered elements with the atomic numbers 21, 31, and 32 were not only found but had properties that Mendeleev had predicted from their positions in the table. These three elements were later named scandium (Sc, 21), gallium (Ga, 31) and germanium (Ge, 32). Mendeleev was officially pronounced to be well clever and the new science of chemistry started to come of age.
It may come as something as a surprise (it usually does) to learn that most elements are metals, non-metals are in the minority. There are a small number of elements between the two that are divided by the black line in the periodic table above that are known as metalloids as they have some of the properties of metals and some of non-metals.
Of all of the elements, 11 are gases, 2 are liquids and all the rest are solids at STP (standard temperature and pressure - 1 atmosphere and 0C). The two liquids are bromine (Br) and mercury (Hg), the gases are hydrogen (H), helium (He), nitrogen (N), oxygen (O), fluorine (F), neon (Ne), chlorine (Cl), argon (Ar), krypton (Kr), xenon (Xe) and radon (Rn).
Some of the groups of groups (vertical) of elements are known by particular names:
 Group
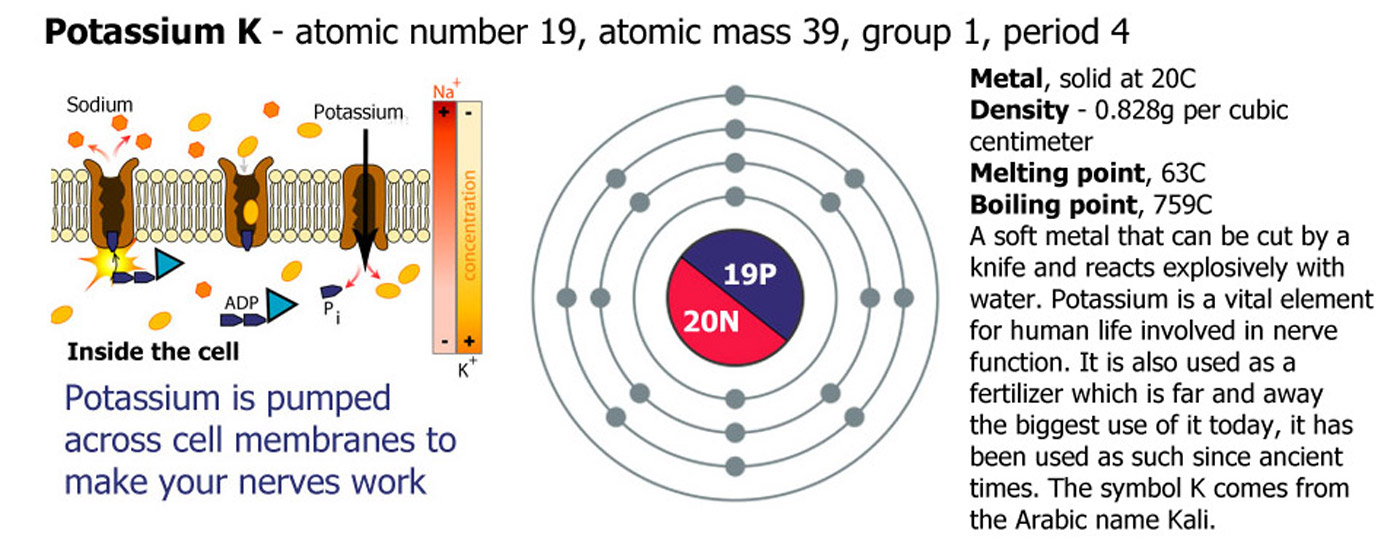
1 - The Alkali Metals
Group
1 - The Alkali Metals
Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs), Francium (Fr). Group 1 are called the alkali metals because they are metals and because they form an alkali solution on contact with water. They increase in reactivity as you go down the group.
Add lithium to water and it reacts quickly floating and fizzing around on the surface producing hydrogen gas and forming strongly alkaline lithium hydroxide solution. Sodium in the same circumstances is a faster reaction and gets hot enough to melt, sodium hydroxide is produced. Potassium is faster still and ignites the hydrogen, often the end of the reaction is a small explosion of the remaining metal. Rubidium reacts violently and immediately with the products spitting out of the container. Cesium is one of the most reactive elements on the periodic table exploding on contact with water, the container will probably be broken. Francium is a highly unstable radioactive element that decays almost as soon as it is formed so no-one has ever been able to drop any in water to see what happens.

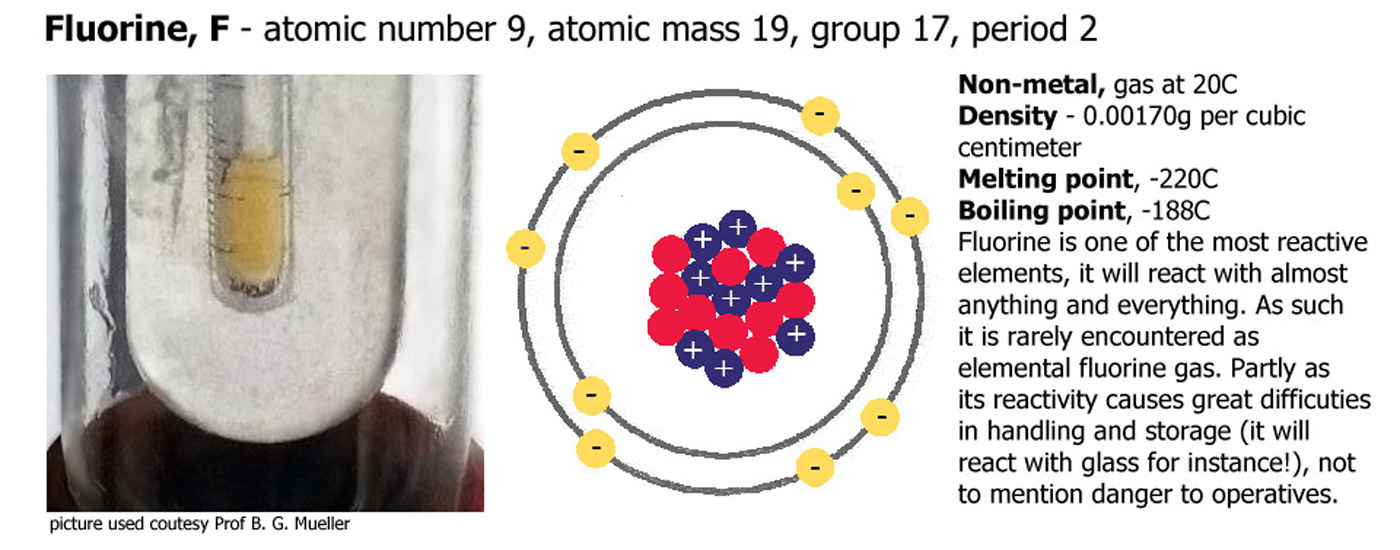
Group 7 (or 17) - The Halogens
Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), Astatine (At). Similar to group 1 but flipped in terms of reactivity, cesium at the bottom of group 1 is one of the most reactive elements in the periodic table, fluorine at the top of group 17 is another exceptionally reactive element. As you go down the group there are a number of trends. Reactivity decreases down the group, the depth of color increases down the group, fluorine is a very pale yellow/green gas, chlorine a deeper color, bromine is an intense orange/brown and iodine a dark grey solid or deep violet when heated to form a gas. Astatine is very unstable and radioactive, it has never been seen, just detected by scientific instruments.
 Group
18 - The Noble Gases
Group
18 - The Noble Gases
Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rd). The noble gases are so called because they have a "noble" temperament being aloof and keeping themselves to themselves. They are colorless, odorless and very unreactive. The boiling pints and melting points increase as you go down the group. One of the best trends are firstly that if you put them in balloons, they go from floating in air to sinking more and more heavily. This however is second to how squeaky or deep they make your voice sound, from the cartoon chipmunk voice you get with helium to the horror-film commentary voice you get with xenon. Radon is radioactive and would give a very high risk of cancer and radiation sickness if you could get enough of it together. If you do this and die, please don't blame me.
Slider images of elements and orbitals used courtesy of Pumbaa, used and re-released under Creative Commons Attribution-Share Alike 2.0 UK: England & Wales license.